Innovative GMP-Monitoring System Tarqvara for the Pharmaceutical Industry with Full Validation Package
The Tarqvara GMP Monitor is an electronic system designed for continuous real-time monitoring and automatic recording of environmental parameters in cleanrooms for pharmaceutical production, warehouses, and healthcare facilities. The system features reliable, validated software, hardware, and calibrated sensors from leading manufacturers such as Vaisala and Dwyer, and is fully GMP-compliant.
Alarm/warning signals are generated when controlled parameters deviate from preset values for longer than the defined delay time. Hygienic signal panels, made from AISI304 stainless steel, equipped with a protected color LCD touch-screen, can display information in any format specified by the client. These panels can be installed in GMP cleanrooms of Grades D, C, and B, as well as in corridors, to inform staff of deviations in controlled parameters through alarms and warnings.
The Tarqvara GMP Monitor ensures fully GMP-compliant recording of parameters, deviations, and system messages. System reports, in either graphic or tabular form, can be printed, saved in PDF format, or exported to an MS Excel-compatible format. The system is equipped with Audit Trail functionality and is supplied with a full validation package in compliance with GMP, GAMP5, and 21 CFR Part 11.
A unique combination of expertise in Validation, GMP, GAMP, and IT/Automation enables our company to develop products with significant advantages over competing systems.
![]()
Client-Tailored Solutions
Tarqvara GMP monitoring systems are developed from the ground up according to customer specifications, allowing for the integration of individual customer requirements related to interface, functionality, report format, appearance/visual design, and other parameters.
![]()
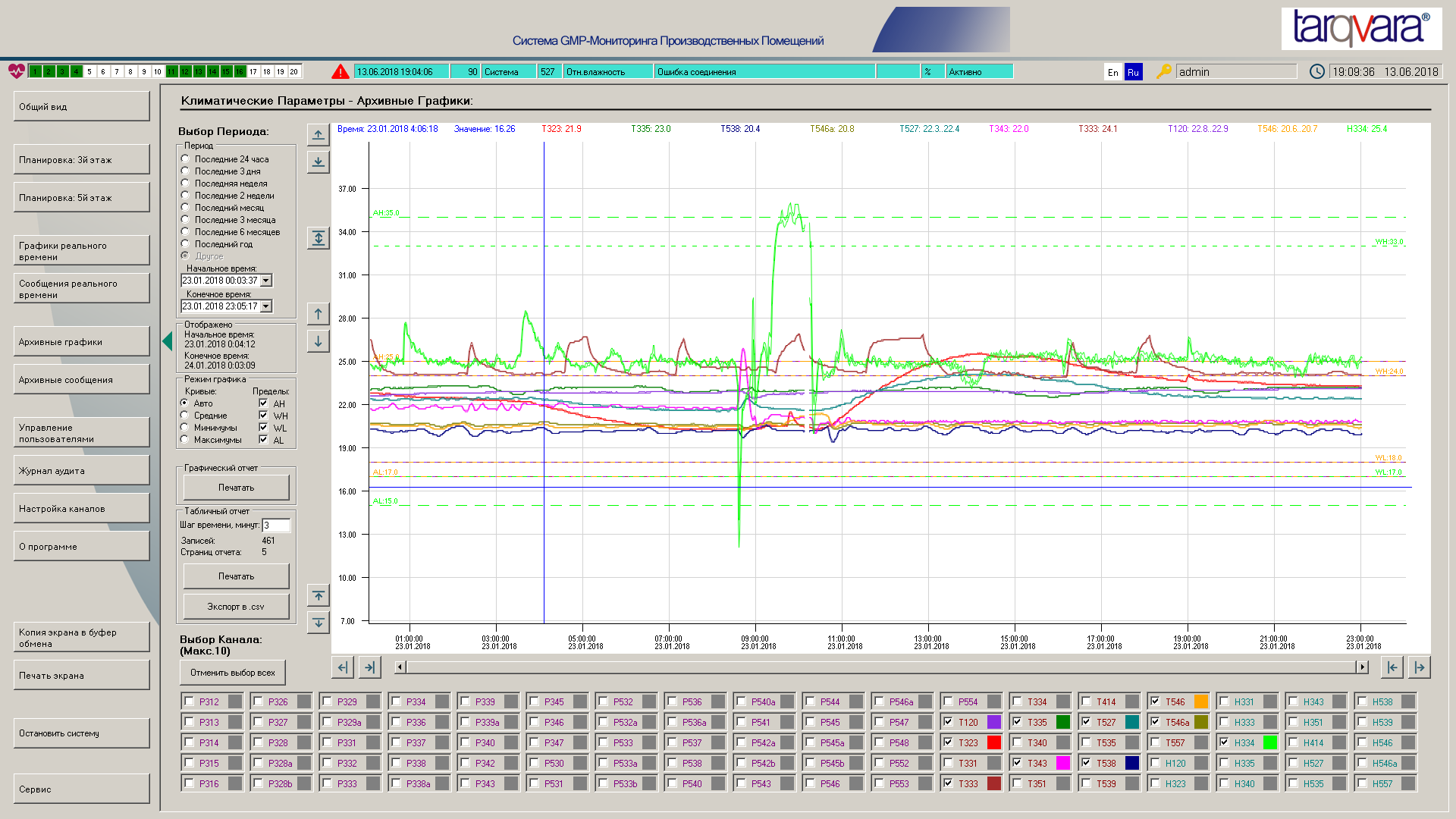
Representative Parameters Recording
In most systems, a single record comprises either a single measurement result or the average of several measurements. A single measurement does not provide information about the process state between measurements, while an average may not capture possible peak values within the period. The Tarqvara GMP Monitor reads parameters once per second, and using the 60 measurements collected each minute, records the first value of the period, along with the average, minimum, and maximum values, and the exact time of the minimum and maximum values. This approach ensures that users receive all representative GMP-critical information.
![]()
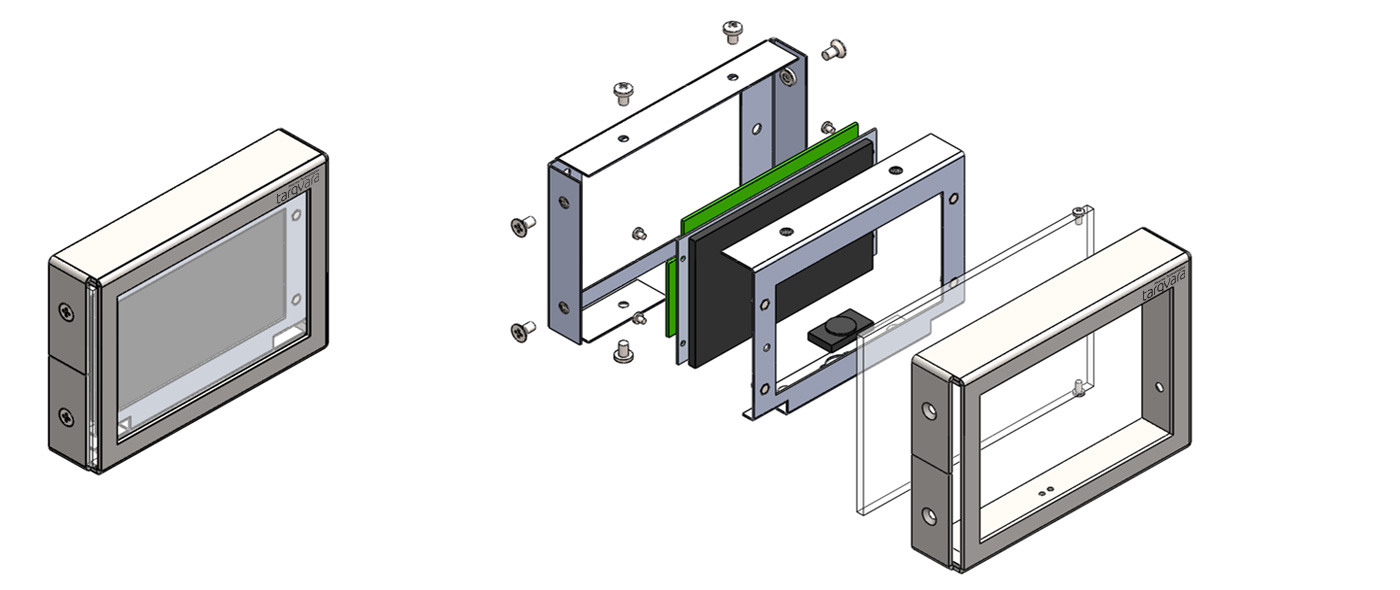
Hygienic design and integration into clean-room structures
All Tarqvara GMP Monitor components installed inside cleanrooms, including sensor mounting elements, differential pressure ports, and alarm panels, feature GMP-compliant design and are made of polished AISI304 stainless steel and other hygienic materials. The equipment and accessories are specially designed for easy and quick installation, integration into standard cleanroom structures, and straightforward maintenance.
![]()
Compact Database
Standard database engines used in most monitoring systems, such as SQL Server, consume significant disk space and are unnecessarily complex for the simple flat data structure of GMP process parameters and messages. The Tarqvara GMP Monitor utilizes its own validated engines for compact and efficient data storage. For example, a 100-channel system with 4-fold data redundancy would generate only about 3 gigabytes of data annually. A database covering 10 years could easily be stored on a single memory stick, and it would take 100 years to fill an average hard disk.
![]()
High Reliability and Audit Trail
Bulky external shells (e.g., WinCC, Desigo, InTouch, etc.) used in many monitoring systems typically include numerous unused components, limiting developer flexibility and making seamless design impossible. This significantly increases the volume of executable program code, places excessive demands on hardware, heightens the risk of failures, and complicates validation. The Tarqvara GMP Monitor has been developed as a compact, directly executable application that only includes components essential to the system. Specific measures have been implemented for both software and hardware to ensure data safety and integrity, as well as to prevent unauthorized data manipulation. A continuous Audit Trail records all user activity and critical system events, such as system time changes.
![]()
High Operating Speed
Displaying long-term curves, generating reports, and working with archive data covering extended periods can be time-consuming in most monitoring systems. In extreme cases, systems may become unresponsive or even crash. The Tarqvara GMP Monitor leverages powerful and efficient hardware system resources for direct access to memory and the file system, resulting in a significant increase in efficiency and operating speed.
![]()
Comprehensive Computer Validation based on GAMP 5
Monitoring system suppliers often provide only standard documents or "white papers" for the development/execution environment (e.g., software shells from Siemens, Wonderware, etc.) as validation documentation, while the actual developers’ project/configuration/program code remains unvalidated and functional tests are undocumented. During the development of the Tarqvara GMP Monitor system, comprehensive computerized system validation (CSV) is conducted, including software validation, installation qualification (IQ), and functional tests, in accordance with GAMP 5 guidelines. Clients receive a complete, compliant validation documentation package, and, if required, additional support during GMP inspections by national and international regulatory bodies, including the FDA.
![]()
Innovative Solutions for Real-Time Monitoring of System Operations
The system performs distributed real-time monitoring of modules operations, ensuring stable system performance even in the event of hardware failure. System modules monitor each other to verify correct operation. If one module fails, it is restarted by the other functioning modules. As a result, a failure in one module does not disrupt the overall system operation, and full functionality is typically restored within 10-20 seconds.
![]()
Reports and Data Export
Reports generated by the Tarqvara GMP Monitor system can include any numerical, textual, or graphical information requested by clients, such as archived values of selected channels for any time period in the form of curves and tables, log records, channel settings, information about channels, registered user accounts, and more. Reports can be printed, saved in PDF format, or exported as an MS Excel-compatible .csv file.
![]()
Efficient and Reliable Network Architecture
In contrast to most monitoring systems, which typically use a centralized architecture, the Tarqvara GMP Monitor employs a distributed architecture. This design significantly reduces the total cabling length, system costs, and ongoing operational expenses. Data from sensors is transmitted in digital form to node gates and the server station, eliminating the influence of external factors such as electromagnetic interference, cable length, and temperature on the transmitted parameters.
See also:
GMP Monitoring Systems
IT Solutions / GAMP / Data Integrity (RDI)
Computerized Systems Validation (CSV)
